Ion exchange resin is an insoluble, cross-linked polymer with a three-dimensional network structure capable of exchanging ions with other ions in a solution. Based on the nature of the exchangeable ions, ion exchange resins can be classified into cation exchange resins and anion exchange resins. Additionally, they can be further categorized into strong acid, weak acid, strong base, and weak base types according to their acidity or alkalinity.
Due to their excellent ion exchange properties, ion exchange resins offer several advantages in pharmaceutical applications, such as:
- Rapid disintegration of drugs
- Controlled drug release
- Reduction of unpleasant drug bitterness
- Improved drug stability
- Enhanced drug dissolution
- Reduction of adverse drug reactions
These properties make ion exchange resins suitable for a variety of pharmaceutical dosage forms, including tablets, capsules, gels, and liquid formulations. Their widespread use in the medical field highlights their versatility and efficacy in enhancing drug performance and patient compliance.
Southwind Technology Pharmaceutical Powdered Resins
To address pharmaceutical needs such as taste masking, controlled release, improved drug stability, and rapid disintegration, Southwind Technology has independently developed and produced ion exchange resins with exceptional performance.
These include:
- Strong cation exchange resins with a polystyrene-divinylbenzene polymer framework.
- Weak cation exchange resins with a polymethacrylic acid-divinylbenzene polymer framework.
Both types of resins have demonstrated outstanding efficacy in various pharmaceutical inhibitors, enhancing drug formulations and functionality.
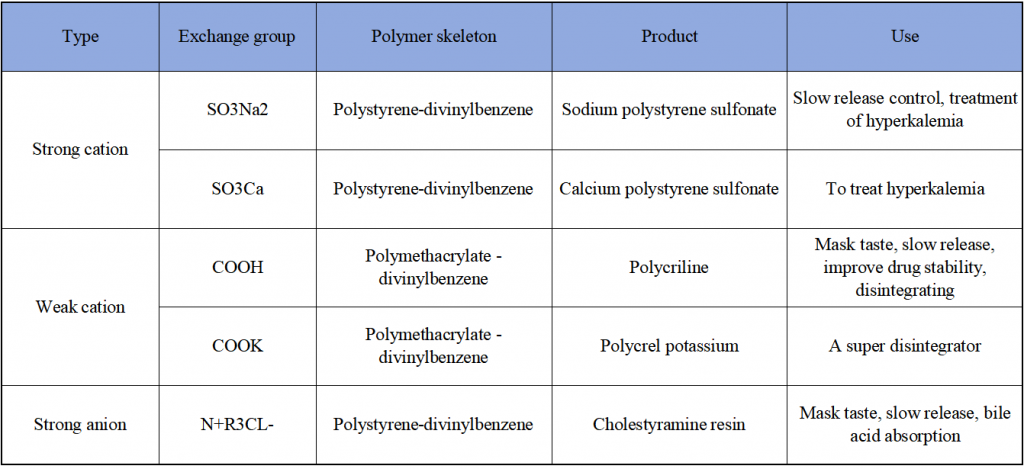
Categories of Powdered Resins
Applications of Ion Exchange Resins in Medical Powdered Resins
Sustained and Controlled Release Formulations
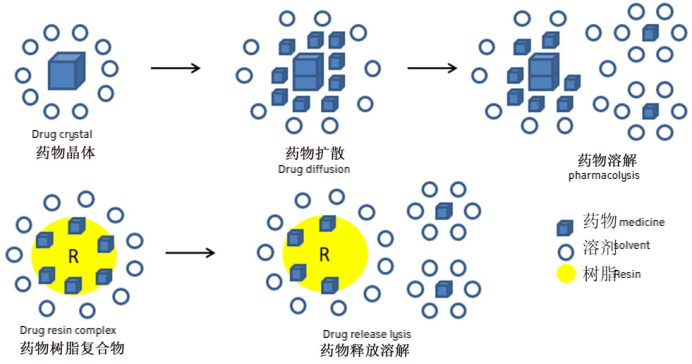
Ion exchange resins can bind with drugs to form drug-resin complexes, where the release of the drug is primarily influenced by endogenous ions such as K⁺, Na⁺, and Cl⁻ in the gastrointestinal tract, and is independent of food intake and pH variations.
Ion exchange is a reversible process. When in contact with highly insoluble substances, ions with the same charge exchange between the liquid and the solid phase. The resin’s structure includes high molecular weight, water-insoluble polymers that are not absorbed by the human body. In the gastrointestinal tract, the resin exchanges ions with gastric or intestinal fluids, releasing the drug from the resin salt for subsequent diffusion.
Mechanism of Cation and Anion Exchange Resins in Forming Drug-Resin Complexes
In the digestive tract, drug-resin complexes can be displaced by the high concentrations of Na⁺, H⁺, or K⁺ ions present in gastric or intestinal fluids. The specific mechanism can be described as follows:
Cation Exchange
- Drug molecules with a positive charge bind to the negatively charged groups in cation exchange resins.
- When exposed to high concentrations of Na⁺, H⁺, or K⁺ in the digestive fluids, the drug is replaced and released into the surrounding medium.
Anion Exchange
- Similarly, negatively charged drug molecules bind to the positively charged groups in anion exchange resins.
- The binding is reversed in the presence of competing anions (e.g., Cl⁻) in the digestive fluids, resulting in drug release.
This process facilitates the sustained or controlled release of drugs, enhancing their therapeutic efficacy and stability.
In the digestive tract, drug-resin complexes can be displaced by high concentrations of Na⁺, H⁺, and K⁺ ions in gastric or intestinal fluids. The specific mechanisms in the stomach and intestine are as follows:
In the Stomach
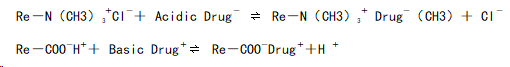
In the Intestine:
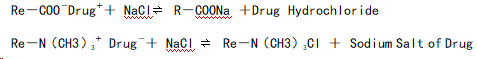
Masking of Taste
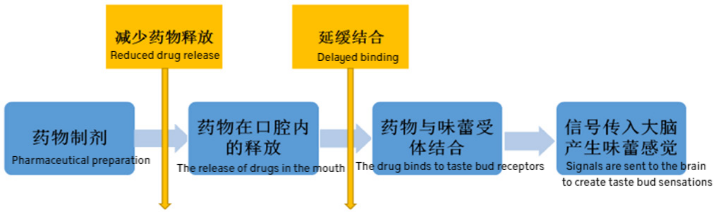
Ion exchange resins are high-molecular cross-linked polymers that contain activatable groups, which can electrostatically adsorb ionic drugs, allowing the drug to enter the resin’s structure. This mechanism helps mask the unpleasant taste of the drug. In saliva with a pH of 6.8, the olfactory sensitivity to the drug in the resin complex is reduced. Since the amount of saliva secreted in the mouth is relatively low and the ion concentration is also low, the resin particles stay in the mouth for a short time during oral administration. Before the drug has a chance to desorb, it enters the stomach. Therefore, this process effectively masks the unpleasant taste of the drug and improves patient compliance.
For improving the taste of bitter drugs, the amount of macroporous ion exchange resin used can vary depending on the drug type. As a general rule, the ratio of the active drug to ion exchange resin should be between 0.1 and 0.75. This technique has been successfully applied to various drugs, including ranitidine and paroxetine.
Reducing Adverse Reactions
By incorporating irritating drugs into the high molecular framework of ion exchange resins, direct contact between the drug and the digestive tract is minimized. This helps to alleviate the adverse reactions that may occur in the upper gastrointestinal tract, such as the mouth, throat, esophagus, and areas with gastric ulcers that come into contact with the drug. The drug is released more gradually, which reduces irritation and improves the overall tolerability of oral formulations.
As a Therapeutic Drug
Ion exchange resins have also been specially identified for use in the treatment of various pathological conditions, such as hyperacidity, ulcers, sodium-potassium depletion, kidney disease, pancreatitis, and cardiac edema. Anion exchange resins have been applied in the treatment of hyperglycemia, while cation exchange resins have been reported for auxiliary treatment in patients with secondary acute renal failure who experience anuria or oliguria. Cholestyramine resin, a strong alkaline anion exchange resin of quaternary ammonium chloride salt, was initially used to control pruritus in patients with elevated plasma bile acid concentrations. This technology has been successfully applied to several drugs, including ranitidine and paroxetine. Sodium polystyrene sulfonate and calcium polystyrene sulfonate have been widely used in clinical settings for the treatment of chronic kidney disease complicated by hyperkalemia.
