The salt separation crystallization processes commonly used in zero wastewater discharge include thermal salt separation, evaporation + freeze crystallization, and nanofiltration + separate evaporation crystallization.
01. Thermal Salt Separation
This method utilizes the solubility differences of sodium sulfate (Na₂SO₄) and sodium chloride (NaCl) at different temperatures and carries out evaporation at varying temperatures. Sodium sulfate is precipitated at high temperatures (80-90°C), and sodium chloride is crystallized at lower temperatures (40-50°C).
- Advantages: Temperature-variable crystallization; the process flow and equipment are relatively simple.
- Disadvantages: Although theoretically both sodium sulfate and sodium chloride can meet the grade-one quality requirements, in practical operation, it is difficult to achieve, and only one qualified product can be produced. Process control is difficult because the solubility of the two salts changes very slightly with temperature. The control of the crystallization of pure salts requires strict precision, and the mother liquor circulation is large.
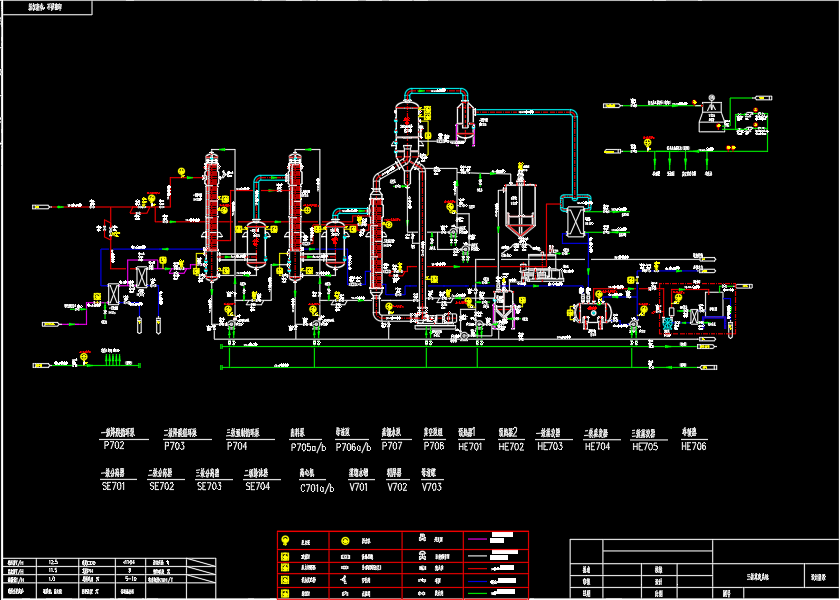
02. Evaporation + Freeze Crystallization
This method takes advantage of the low solubility of decahydrate sodium sulfate at low temperatures (for example, at -5°C, the sodium sulfate content at the common saturation point of sodium chloride and sodium sulfate is 0.71%, while the sodium chloride content is 25.06%). Sodium sulfate is separated by freezing at low temperatures, and the frozen mother liquor (with a mass ratio of sodium chloride to sodium sulfate reaching 35:1) is evaporated and crystallized at high temperatures to obtain sodium chloride.
- Advantages: Good adaptability to concentrated brine with varying ratios of sodium sulfate and sodium chloride; flexible operation; better tolerance for high hardness and high silica systems with nanofiltration membranes; relatively simple equipment with low investment and operational costs.
- Disadvantages: Poor control of the freezing temperature of the mother liquor, which significantly impacts fluctuations in the composition of the mother liquor. All impurity ions or COD in the system accumulate in the mother liquor, which can affect the quality of sodium chloride. This is a generally effective salt separation process.
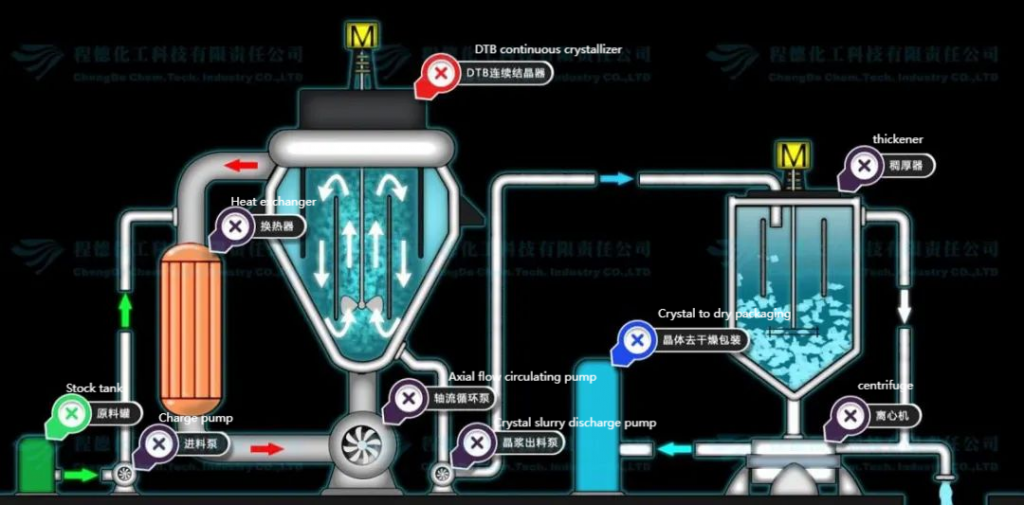
03. Nanofiltration + Separate Evaporation Crystallization
This method utilizes nanofiltration to separate monovalent and divalent ions in concentrated brine. The monovalent ions, primarily sodium chloride, contain potassium, nitrate, and a small amount of sulfate, and are evaporated and crystallized to obtain purer sodium chloride. The divalent ion solution primarily contains sodium sulfate and some sodium chloride, with most organic substances concentrated in the divalent ion solution (via advanced oxidation), which is then evaporated and crystallized to produce anhydrous sodium sulfate.
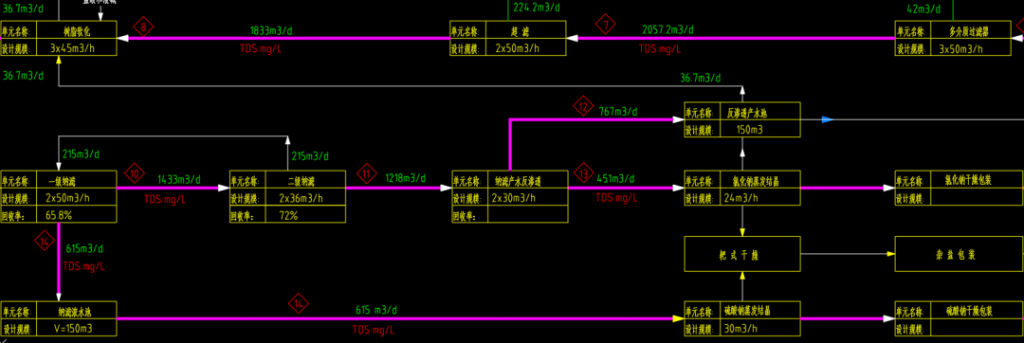
- Advantages: High adaptability to fluctuations in influent water quality. Nanofiltration helps maintain stable water quality for the crystallization unit, ensuring stable salt quality; complete separation of sodium sulfate and sodium chloride; minimal discharge of mother liquor; maximum resource utilization of crystallized salts.
- Disadvantages: Higher equipment investment, shorter lifespan of nanofiltration membranes, and higher operational costs. This method is more suitable for concentrated brine with lower COD and hardness and higher sodium chloride content.
