Industrial wastewater includes production wastewater, process sewage, and cooling water, often carrying industrial raw materials, intermediates, by-products, and pollutants. Here’s a summary of common treatment methods:

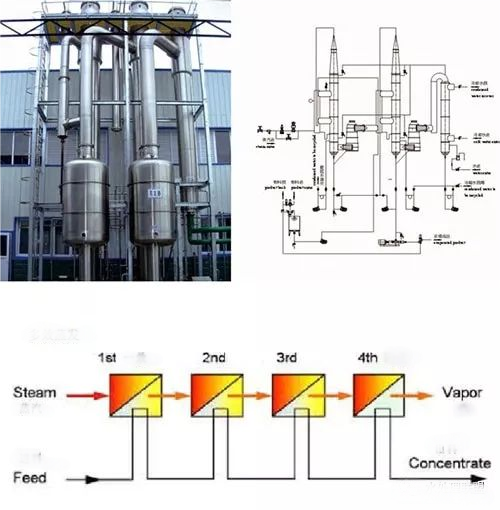
1. Multi-Effect Evaporation Crystallization Technology
Used for saline industrial wastewater treatment, this method involves low-temperature multi-effect evaporation and crystallization. Wastewater is separated into desalinated water and concentrated slurry. Crystallizable substances are incinerated into inorganic residue, while non-crystallizable organic waste is turned into solid residue using rotary evaporators. Desalinated water can be reused in production. The system efficiently utilizes secondary steam to reduce costs and improve economic efficiency.
2. Biological Methods
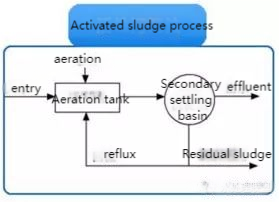
(1) Traditional Activated Sludge Process
This aerobic method effectively removes biodegradable organic matter and some nitrogen and phosphorus. It’s suited for stable, high-quality wastewater but struggles with fluctuating water quality, large oxygen demands, and requires significant space.
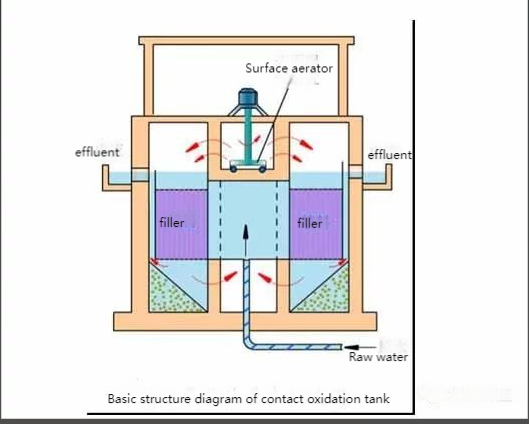
(2) Biological Contact Oxidation
This process uses microorganisms attached to solid surfaces (biomembranes) to treat organic wastewater. It combines the advantages of activated sludge and biomembrane methods, offering high efficiency, adaptability to shock loads, low sludge production, simple operation, and stable water quality. It also removes phosphorus and decomposes hard-to-treat organics, serving as a tertiary treatment.

3. SBR Process (Sequencing Batch Reactor)
This batch operation method includes five stages: inflow, reaction, sedimentation, discharge, and idle. It features simple design, small footprint, low investment, high efficiency, and flexibility. It effectively removes nitrogen and phosphorus while handling shock loads. However, it requires flow regulation for high influent volumes, increasing costs.
4. MBR Process (Membrane Bioreactor)
This method combines membrane filtration with activated sludge. Wastewater is filtered through a unique MBR membrane module after biological treatment. It delivers compact design, superior water quality, minimal sludge, and efficient removal of ammonia and refractory organics. However, its high cost, membrane fouling, energy demand, and operational complexity are challenges.
Each method has distinct advantages and limitations, making their selection dependent on specific wastewater characteristics and treatment goals.
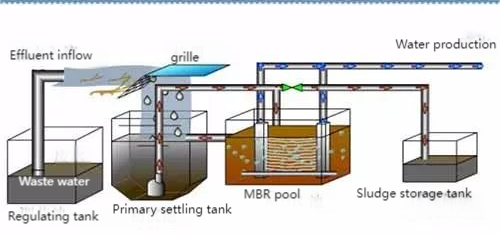
05. Electrolysis Process
Electrolysis leverages the high conductivity of saline wastewater for redox reactions in an electrolytic cell. This process generates insoluble compounds, which can be removed via sedimentation or gas flotation, while some organics are oxidized to harmless gases, lowering COD levels. Sodium chloride in the solution undergoes secondary reactions, producing bleaching agents like hypochlorite and chlorate, aiding in organic pollutant degradation. While promising, challenges like high energy consumption and operational costs limit its application, keeping it in the research phase for saline wastewater treatment.
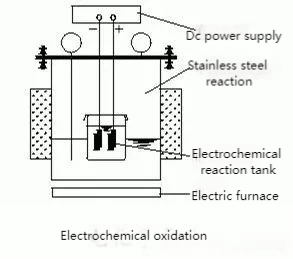
06. Ion Exchange Method
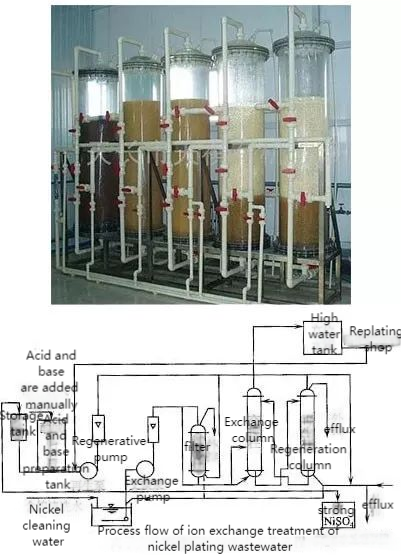
Ion exchange involves the substitution of ions in solution with those on an insoluble polymer (resin).
- Process: Wastewater passes through a cation exchange column, where positive ions (e.g., Na⁺) are replaced by H⁺. Then, an anion exchange column removes negative ions (e.g., Cl⁻) by exchanging them for OH⁻, achieving desalination.
This method is efficient for specific ion removal but requires regular resin regeneration and handling of regenerant waste.
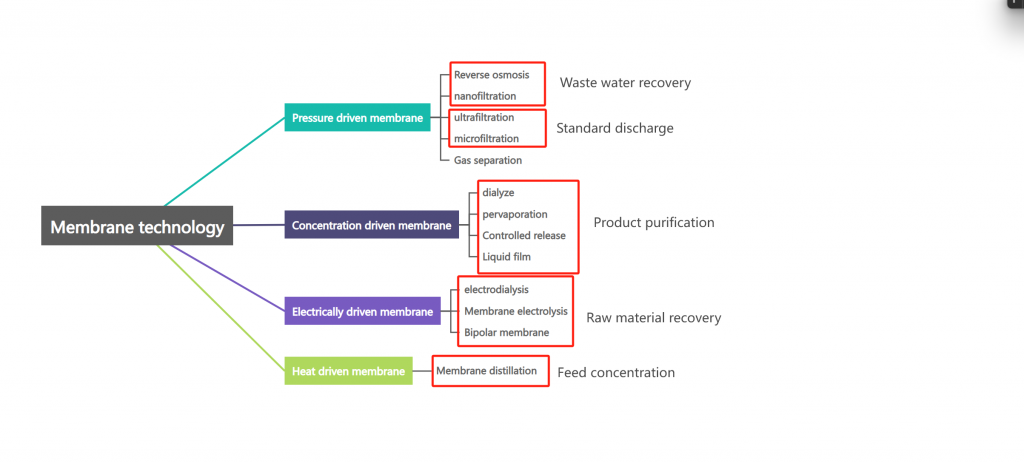
07. Membrane Separation Technology
Membrane technologies exploit the selective permeability of membranes to separate, purify, or concentrate substances.
- Types:
- Ultrafiltration (UF) and Microfiltration (MF): Efficient for SS and colloid COD removal but not for salts.
- Electrodialysis (ED) and Reverse Osmosis (RO): Highly effective for desalination, with RO being the most widely used.
- Challenges: High costs, membrane fouling, scaling, and maintenance issues. Advances in membrane manufacturing are expected to expand applications in wastewater treatment.
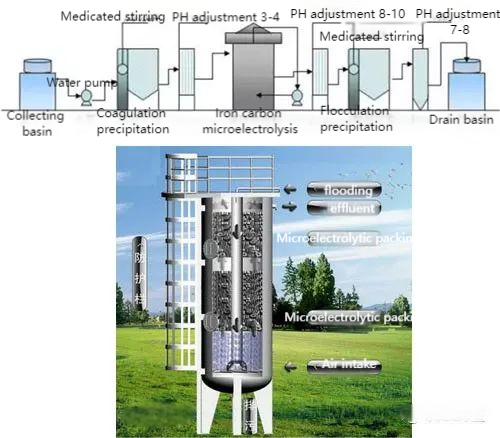
08. Iron-Carbon Micro-Electrolysis (ICME)
ICME, also known as internal electrolysis, uses Fe/C micro-galvanic cells to treat wastewater.
- Mechanism: Immersed iron filings in wastewater form numerous galvanic cells. Adding coke enhances the galvanic effect, accelerating reactions like oxidation, coagulation, and floc adsorption.
- Advantages:
- Broad applicability (e.g., textile, pesticide, heavy metal, petrochemical wastewater).
- Cost-effective and low-maintenance.
- Eco-friendly (“treating waste with waste”), using scrap iron as a resource.
ICME is widely adopted and effective for treating refractory wastewater.
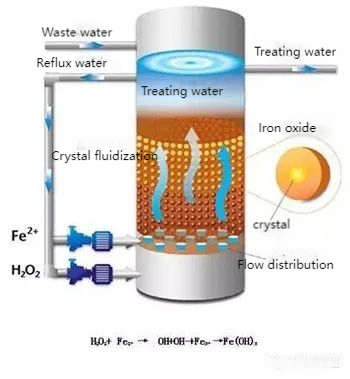
09. Fenton and Fenton-like Oxidation
The Fenton process uses Fe²⁺ to catalyze H₂O₂ decomposition, generating hydroxyl radicals (˙OH) that oxidize organic pollutants.
- Challenges: Requires long reaction times and large reagent doses, with excess Fe²⁺ potentially increasing COD and causing secondary pollution.
- Improvements: Incorporating UV/visible light or substituting Fe²⁺ with other transition metals enhances oxidation efficiency, reduces reagent use, and lowers costs.
- Applications: Effective as a standalone or supplementary treatment, often paired with coagulation, adsorption, or biological methods for pre-treatment or advanced treatment of refractory wastewater.